
Regulatory Affairs Manager CMC (m/f/x)
Wörwag Pharma GmbH & Co. KG • Böblingen
-
Full-time
Böblingen
Wörwag Pharma – a medium-sized, internationally active, family-owned pharmaceutical company headquartered in Böblingen near Stuttgart, Germany, which recognized and scientifically proved the importance of biofactors very early on. For more than 50 years, we have been fighting the civilization diseases of our time - preventive, accompanying, healing. Our slogan "Getting closer helping better" is not just a promise to doctors, pharmacists and patients. It is an expression of our colorful corporate culture and our collegial cooperation among our now 1,200 employees worldwide.
Become a part of our team in our headquarter in Böblingen (near Stuttgart) because we are looking for you as a
Regulatory Affairs Manager CMC (m/f/x)
These tasks await you
- Ensuring the quality of documentation for marketing authorisations in terms of content and regulatory requirements, with a focus on pharmaceutical quality (Module 3, Module 2.3)
- Independent preparation/authoring and updating of CMC documentation (Module 3 and Module 2.3) of international marketing authorisation dossiers / core dossiers
- Timely provision of CMC relevant documentation to RA-M1 managers for global new submissions and renewals, as well as for quality relevant variations (national, European harmonized, other harmonized and global)
- Timely support in response to complaints/deficiencies about pharmaceutical quality
- Preparation of administrative approval documents, as long as they relate to the content of Module 3
- Regulatory assessment of module 3 and module 2.3 taking into account the manufacturing documentation and current guidelines in the process of change evaluation
- Regulatory support of cross-departmental projects (e.g. in the case of changes in production, changes in manufacturer, technical transfer, changes in the composition of medicinal products) for the European and global markets
- Consultation on approval-related issues within the company and from the authorities
- Regulatory assessment of ASMFs
- Training of regulatory apprentices in terms of regulatory requirements and working practices
- Development and optimization of internal regulatory processes
What you bring with you
- Degree in chemistry, pharmacy or a comparable natural science
- At least 2 years of professional experience in regulatory affairs
- Very good knowledge of the legal regulations concerning drug registration
- Very good knowledge of the technical and content requirements for authoring of a marketing authorisation dossier
- Readiness to cooperate within the Regulatory Affairs Department (within CMC team and with other teams), with in-house departments (Development, Quality, Purchasing, GMA) and contract manufacturers, as well as with external contractors
- Independent, structured and integrative work, assertiveness, ability to deal with conflicts, resilience, reliability
- Very good PC knowledge (MS-Office)
- Very good knowledge of English
- Good knowledge of process and project management
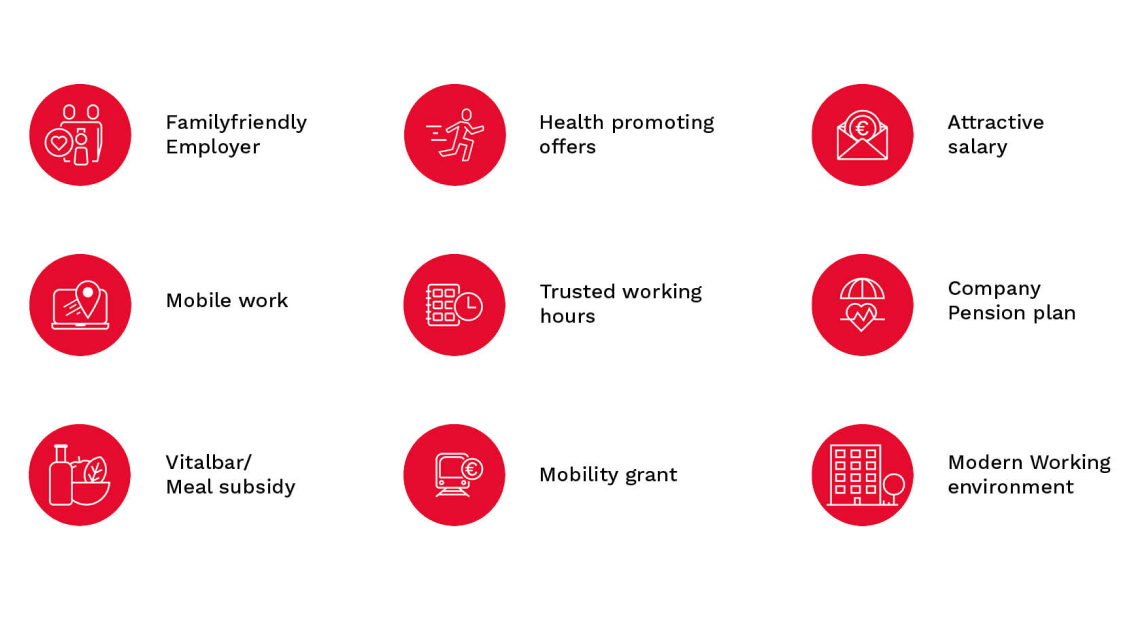
We offer you
- A dynamic, international environment with the opportunity to take on responsibility quickly and extensively, to contribute experience and knowledge and to implement ideas
- Varied challenges, as well as exciting projects with a strong team spirit
- Compensation in line with the market and your performance, as well as additional benefits which can be used flexibly according to your needs, e.g.,
- Subsidy for direct insurance
- Individual compensation options for days off, pension provisions or payout
- Bonago vouchers for special occasions
- Support for your general well-being and/or work-life balance
- Health-promoting measures such as discounted conditions on over-the-counter (OTC) pharmacy products or health days
- Flexible trust working hours as well as the possibility to work mobile up to 75% of your working time
- Central company headquarters with free parking facilities, good public transport connections as well as mobility allowances
- A wide range of opportunities for personal and professional development
- Regular employee events
We are looking forward to receiving your application!
That´s us

Our benefits
Working at Wörwag Pharma
What our employees say

Alexander Bürger
Send email E-Mail copied! Copy E-Mail?
Wörwag Pharma GmbH & Co. KG
Flugfeld-Allee 24
71034 Böblingen





